- Some properties of the rare-earth elements (values recommended by Ames Laboratory) part I
-
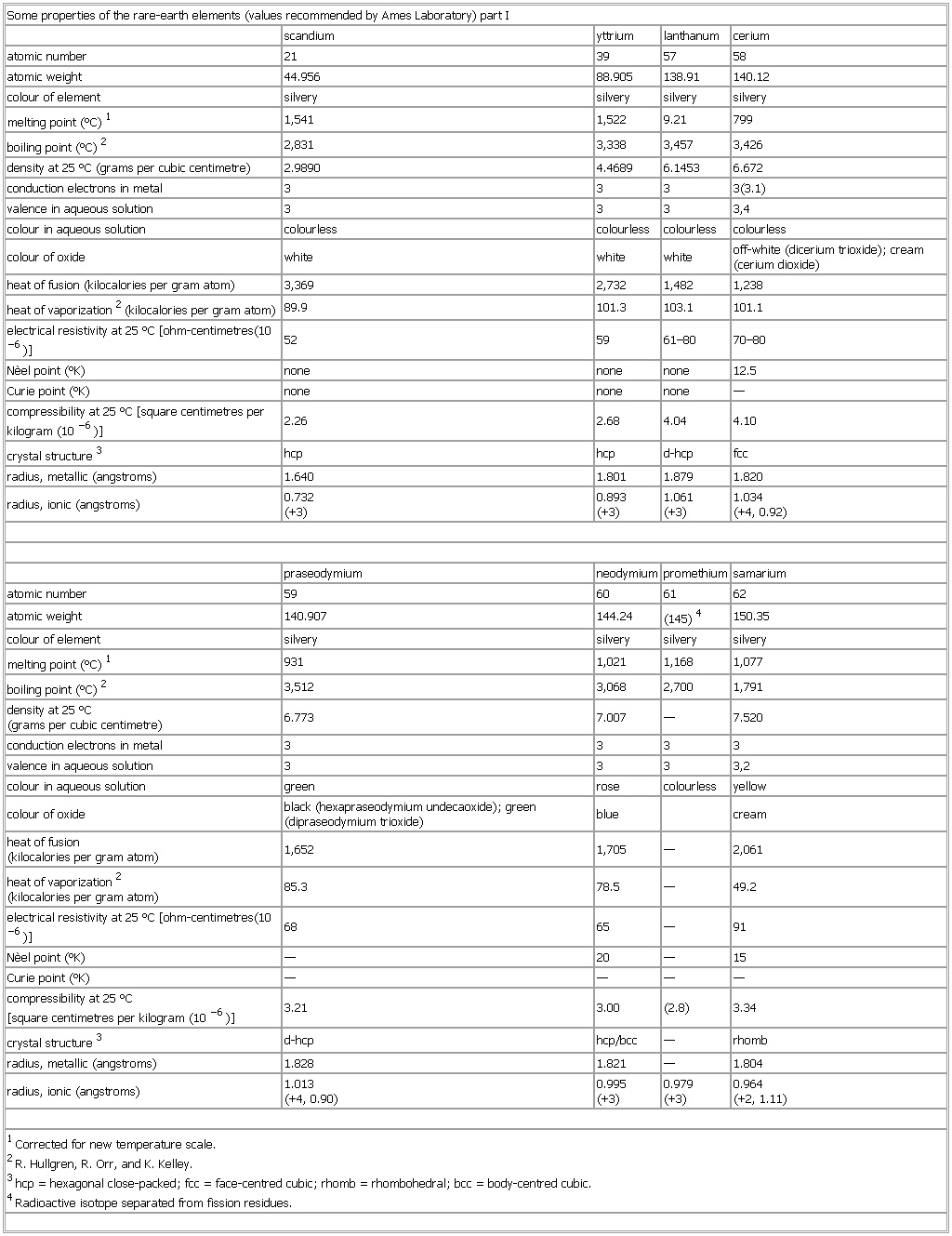
▪ TableSome properties of the rare-earth elements (values recommended by Ames Laboratory) part Iscandium yttrium lanthanum ceriumatomic number 21 39 57 58atomic weight 44.956 88.905 138.91 140.12colour of element silvery silvery silvery silverymelting point (°C)1 1,541 1,522 9.21 799boiling point (°C)2 2,831 3,338 3,457 3,426density at 25 °C (grams per cubic centimetre) 2.9890 4.4689 6.1453 6.672conduction electrons in metal 3 3 3 3(3.1)valence in aqueous solution 3 3 3 3,4colour in aqueous solution colourless colourless colourless colourlesscolour of oxide white white white off-white (dicerium trioxide); cream (cerium dioxide)heat of fusion (kilocalories per gram atom) 3,369 2,732 1,482 1,238heat of vaporization2 (kilocalories per gram atom) 89.9 101.3 103.1 101.1electrical resistivity at 25 °C [ohm-centimetres(10−6)] 52 59 61–80 70–80Nèel point (°K) none none none 12.5Curie point (°K) none none none —compressibility at 25 °C [square centimetres per kilogram (10−6)] 2.26 2.68 4.04 4.10crystal structure3 hcp hcp d-hcp fccradius, metallic (angstroms) 1.640 1.801 1.879 1.820radius, ionic (angstroms) 0.732(+3) 0.893(+3) 1.061(+3) 1.034(+4, 0.92)praseodymium neodymium promethium samariumatomic number 59 60 61 62atomic weight 140.907 144.24 (145)4 150.35colour of element silvery silvery silvery silverymelting point (°C)1 931 1,021 1,168 1,077boiling point (°C)2 3,512 3,068 2,700 1,791density at 25 °C(grams per cubic centimetre) 6.773 7.007 — 7.520conduction electrons in metal 3 3 3 3valence in aqueous solution 3 3 3 3,2colour in aqueous solution green rose colourless yellowcolour of oxide black (hexapraseodymium undecaoxide); green (dipraseodymium trioxide) blue creamheat of fusion(kilocalories per gram atom) 1,652 1,705 — 2,061heat of vaporization2(kilocalories per gram atom) 85.3 78.5 — 49.2electrical resistivity at 25 °C [ohm-centimetres(10−6)] 68 65 — 91Nèel point (°K) — 20 — 15Curie point (°K) — — — —compressibility at 25 °C[square centimetres per kilogram (10−6)] 3.21 3.00 (2.8) 3.34crystal structure3 d-hcp hcp/bcc — rhombradius, metallic (angstroms) 1.828 1.821 — 1.804radius, ionic (angstroms) 1.013(+4, 0.90) 0.995(+3) 0.979(+3) 0.964(+2, 1.11)1Corrected for new temperature scale.2R. Hullgren, R. Orr, and K. Kelley.3hcp = hexagonal close-packed; fcc = face-centred cubic; rhomb = rhombohedral; bcc = body-centred cubic.4Radioactive isotope separated from fission residues.See as table:
* * *
Universalium. 2010.
