Equilibrium constants for the formation of various nickel-amine complexes
- Equilibrium constants for the formation of various nickel-amine complexes
-
Equilibrium constants for the formation
of various nickel-amine complexes
n amine (L) equilibrium constant
KL, M−1*
1 NH3 5 × 102
2 NH2CH2CH2NH2 4 × 107
3 NH2CH2CH2NHCH2CH2NH2 5 × 1010
4 NH2CH2CH2NHCH2CH2NHCH2CH2NH2 1 × 1014
*M is molar concentration.
See as table: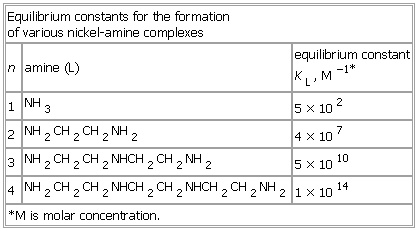
* * *
Universalium.
2010.
Look at other dictionaries:
coordination compound — Chem. complex (def. 10). Also called coordination complex. * * * ▪ chemistry Introduction any of a class of substances with chemical structures in which a central metal atom is surrounded by nonmetal atoms or groups of atoms, called ligands… … Universalium
catalysis — catalytic /kat l it ik/, adj., n. catalytical, adj. catalytically, adv. /keuh tal euh sis/, n., pl. catalyses / seez /. 1. Chem. the causing or accelerating of a chemical change by the addition of a catalyst. 2. an action between two or more… … Universalium
Pyridine — Pyridine … Wikipedia

