- Discovery of the Transuranium Elements, Table
-
▪ TableTable 27: Discovery of the Transuranium Elementsatomicnumber element symbol atomicweight discoverers anddate of discovery source of firstamount (half-life)93 neptunium Np 237*† E.M. McMillan and P.H. Abelsonat the University of California,Berkeley, 1940 irradiation of uranium with neutrons23892U + 10n ——> 23992U + gamma rayß-23992U ——> 23993Np (2.36 days) 1944, 23793Np (2,140,000 years)L.B. Magnusson and T.J.La Chappelle at theUniversity of Chicago94 plutonium Pu 239†244* G.T. Seaborg, E.M. McMillan,J.W. Kennedy, and A.C. Wahlat the University of California,Berkeley, 1940 bombardment of uranium with deuterons23892U + 21H ——> 23893Np + 210nß-23893Np ——> 23894Pu (87.7 years) 1942, 23994Pu (24,100 years)B.B. Cunningham andL.B. Werner at theUniversity of Chicago95 americium Am 241†243* G.T. Seaborg, R.A. James, L.O.Morgan, and A. Ghiorso atthe University of Chicago, 1944–45 irradiation of plutonium with neutrons23994Pu + 10n ——> 24094Pu + gamma ray24094Pu + 10n ——> 24194Pu + gamma rayß-24194Pu ——> 24195Am (432 years) 1945, 24195Am (432 years)B.B. Cunningham at theUniversity of Chicago96 curium Cm 244†247* G.T. Seaborg, R.A. James, andA. Ghiorso at the Universityof Chicago, 1944 bombardment of plutonium with helium ions23994Pu + 42He ——> 24296Cm (163 days) + 10n 1947, 24296Cm (163 days)L.B. Werner andI. Perlman at theUniversity of California,Berkeley97 berkelium Bk 247*249† S.G. Thompson, A. Ghiorso, andG.T. Seaborg at the Universityof California, Berkeley, 1949 bombardment of americium with helium ions24195Am + 42He —> 24397Bk (4.5 hours) + 210n 1958, 24997Bk (320 days)S.G. Thompson and B.B.Cunningham at theUniversity of California,Berkeley98 californium Cf 251*252† S.G. Thompson, K. Street, Jr.,A. Ghiorso, and G.T. Seaborgat the University of California,Berkeley, 1950 bombardment of curium with helium ions24296Cm + 42He ——> 24598Cf (45 min) + 10n 1958, 249, 250, 251, 252CfB.B. Cunningham and S.G.Thompson at theUniversity of California,Berkeley99 einsteinium Es 253†252* scientists at the University ofCalifornia, Berkeley; ArgonneNational Laboratory, Illinois;and Los Alamos ScientificLaboratory, New Mexico; 1952 found in the debris from the first thermonuclearexplosion, nicknamed "Mike," engineered bythe Los Alamos Scientific Laboratory, New Mexico,on Nov. 1, 1952, at Enewetak atoll, MarshallIslands; successive neutron captures by uranium-238and the subsequent ß- decay of the capture productsresulted in 25399Es (20.5 days) 1961, 25399Es (20.5 days)B.B. Cunningham, J.C.Wallmann, L. Phillips,and R.C. Gatti at theUniversity of California,Berkeley100 fermium Fm 257*† scientists at the University ofCalifornia, Berkeley; ArgonneNational Laboratory, Illinois;and Los Alamos ScientificLaboratory, New Mexico; 1953 found in the debris from the first thermonuclearexplosion, nicknamed "Mike," engineered bythe Los Alamos Scientific Laboratory, New Mexico,on Nov. 1, 1952, at Enewetak atoll, MarshallIslands; successive neutron captures by uranium-238and the subsequent ß- decay of the capture productsresulted in 255100Fm (20.1 hours) . . .101 mendelevium Md 258* A. Ghiorso, B.G. Harvey, G.R.Choppin, S.G. Thompson, andG.T. Seaborg at the Universityof California, Berkeley, 1955 bombardment of einsteinium with helium ions25399Es + 42He ——> 256101Md (1.3 hours) + 10n . . .102 nobelium No 259* A. Ghiorso, T. Sikkeland,J.R. Walton, and G.T. Seaborgat the University of California,Berkeley, 1958E.D. Donets, V.A. Schegolev, andV.A. Ermakov at the JointInstitute for Nuclear Researchat Dubna, Russia, 1964 bombardment of curium with carbon ions24696Cm + 126C ——> 254102No (55 sec) + 410nincorrectly identified as 254102No)bombardment of curium with carbon ions24896Cm + 126C ——> 256102No (3.3 sec) + 410n . . .. . .103 lawrencium Lr 260* A. Ghiorso, T. Sikkeland,A.E. Larsh, and R.M. Latimerat the University of California,Berkeley, 1961E.D. Donets, V.A. Schegolev, andV.A. Ermakov at the JointInstitute for Nuclear Researchat Dubna, Russia, 1965bombardment of a mixture of californium isotopeswith boron-10 ions and baron-11 ions24998Cf 210n25098Cf +105B ——> 258103Lr + 310n25198Cf +115B 410n25298Cf 510n(the product was incorrectly identified as 257103Lr in1961; the isotope discovered was later proved to be258103Lr; 3.9 sec)bombardment of americium with oxygen ions24395Am + 188O ——> 256103Lr (28 sec) + 510n . . .. . .104 unnilquadium‡rutherfordium** UnqRf 261* A. Ghiorso, M. Nurmia, J. Harris,K. Eskola, and P. Eskola at theUniversity of California,Berkeley, 1969G.N. Flerov, Y.T. Oganessian,Y.V. Lobanov, V.I. Kuznetsov,V.A. Druin, V.P. Perelygin,K.A. Gavrilov, S.P. Tretyakova,and V.M. Plotko at the JointInstitute for Nuclear Researchat Dubna, Russia, 1964 bombardment of californium with carbon ions24998Cf + 126C ——> 257104 (4.7 sec) + 410n24998Cf + 136C ——> 259104 (3.1 sec) + 310nbombardment of plutonium with neon ions24294Pu + 2210Ne ——> 260104 + 410n(identification of the isotope produced has not beenverified; later research has raised questions about someof the assumptions on which the original discoveryclaim was based) . . .. . .105 unnilpentium‡hahniumdubnium** UnpHaDb 262* A. Ghiorso, M. Nurmia, K. Eskola,J. Harris, and P. Eskola at theUniversity of California,Berkeley, 1970G.N. Flerov, V.A. Druin,A.G. Demin, Y.V. Lobanov,N.K. Skokelev, G.N. Akap'ev,B.V. Fefilov, I.V. Kolesov,K.A. Gavrilov, Y.P. Kharitonov,and L.P. Chelnokov at theJoint Institute for NuclearResearch at Dubna, Russia, 1968 bombardment of californium with nitrogen ions24998Cf + 157N ——> 260105 (1.5 sec) + 410nbombardment of americium with neon ions24395Am + 2210Ne ——> 260105 + 510n24395Am + 2210Ne ——> 261105 + 410n(the Dubna scientists reported observing alpha-particle-emitting isotopes of element 105, buttheir findings were generally considered inconclusive;in 1970 Dubna scientists reported observing the nuclide261105, which decayed by spontaneous fission) . . .. . .106 unnilhexium‡seaborgium** UnhSg 266* A. Ghiorso, J.M. Nitschke,J.R. Alonso, C.T. Alonso,M. Nurmia, G.T. Seaborg,E.K. Hulet, and R.W. Lougheedat the University of California,Berkeley, 1974Y.T. Oganessian, Y.P. Tretyakov,A.S. Iljinov, A.G. Demin,A.A. Pleve, S.P. Tretyakova,V.M. Plotko, M.P. Ivanov,N.A. Danilov, Y.S. Korotkin,and G.N. Flerov at the JointInstitute for Nuclear Researchat Dubna, Russia, 1974 bombardment of californium with oxygen ions24998Cf + 188O ——> 263106 (0.8 sec) + 410nbombardment of lead with chromium ions20782Pb + 5424Cr ——> 259106 + 210n20882Pb + 5424Cr ——> 259106 + 310n(later research showed that the observed spontaneousfission was due to 256, 255104, not element 106). . .. . .107 unnilseptium‡nielsbohriumbohrium** UnsNsBh 262* G. Münzenberg, S. Hofmann,F.P. Hessberger, W. Reisdorf,K.H. Schmidt, J.R.H. Schneider,W.F.W. Schneider, P. Armbruster,C.C. Sahm, and B. Thuma at theInstitute for Heavy Ion Researchat Darmstadt, Ger., 1981 bombardment of bismuth with chromium ions20983Bi + 5424Cr ——> 262107 (0.1 sec) + 10n . . .108 unniloctium‡hassium** UnoHs 265* G. Münzenberg, P. Armbruster,H. Folger, F.P. Hessberger,S. Hofmann, J. Keller,K. Poppensieker, W. Reisdorf,K.H. Schmidt, H.J. Schött,M.E. Leino, and R. Hingmannat the Institute for Heavy IonResearch at Darmstadt, Ger., 1984 bombardment of lead with iron ions20882Pb + 5826Fe ——> 265108 (2 ms) + 10n . . .109 unnilennium‡meitnerium** UneMt 266* G. Münzenberg, P. Armbruster,F.P. Hessberger, S. Hofmann,K. Poppensieker, W. Reisdorf,K. Schneider, K.H. Schmidt,C.C. Sahm, and D. Vermeulen atthe Institute for Heavy IonResearch at Darmstadt,Ger., 1982 bombardment of bismuth with iron ions20983Bi + 5826Fe ——> 266109 (3.4 ms) + 10n . . .110 ununnilium‡ Uun 271* S. Hofmann, V. Ninov,F.P. Hessberger, P. Armbruster,H. Folger, G. Münzenberg,H.J. Schött, A.G. Popeko,A.V. Yeremin, A.N. Andreyev,S. Saro, R. Janik, and M. Leinoat the Institute for Heavy IonResearch at Darmstadt, Ger., 1994A. Ghiorso, D. Lee, L.P.Somerville, W. Loveland,J.M. Nitschke, W. Ghiorso,G.T. Seaborg, P. Wilmarth,R. Leres, A. Wydler, M. Nurmia,K. Gregorich, R. Gaylord,T. Hamilton, N.J. Hannink,D.C. Hoffman, C. Jarzynski,C. Kacher, B. Kadkhodayan,S. Kreek, M. Lane, A. Lyon,M.A. McMahan, M. Neu,T. Sikkeland, W.J. Swiatecki,A. Türler, J.T. Walton,and S. Yashita at the Universityof California, Berkeley, 1991–94Y.A. Lazarev, Y.V. Lobanov,Y.T. Oganessian, V.K. Utyonkov,S. Iliev, V.G. Subbotin,A.M. Sukhov, G.V. Buklanov,B.N. Gikal, V.B. Kutner,A.N. Mezentsev, K. Subotic,I.F. Wild, R.W. Lougheed,and K.J. Moody at the JointInstitute for Nuclear Researchat Dubna, Russia, and LawrenceLivermore National Laboratory,California, 1994– bombardment of lead with nickel ions20882Pb + 6228Ni ——> 269110 (0.17 ms) + 10n20882Pb + 6428Ni ——> 271110 (1.4 ms) + 10nbombardment of bismuth with cobalt ions5927Co + 20983Bi ——> 267110 (4 ms) + 10n(there is some evidence that one atom of 267110 mayhave been created in 1991)bombardment of plutonium with sulfur ions24494Pu + 3416S ——> 273110 (0.3 ms) + 510n(one decay chain attributable to 273110 has beenidentified, but data is still being analyzed) . . .. . .. . .111 unununium‡ Uuu 272* S. Hofmann, V. Ninov,F.P. Hessberger, P. Armbruster,H. Folger, G. Münzenberg,H.J. Schött, A.G. Popeko,A.V. Yeremin, A.N. Andreyev,S. Saro, R. Janik, and M. Leinoat the Institute for Heavy IonResearch at Darmstadt, Ger., 1994 bombardment of bismuth with nickel ions20983Bi + 6428Ni ——> 272111 (1.5 ms) + 10n . . .112 ununbium‡ Uub 277* S. Hofmann, V. Ninov,F.P. Hessberger, P. Armbruster,H. Folger, G. Münzenberg,H.J. Schött, A.G. Popeko,A.V. Yeremin, S. Saro,R. Janik, and M. Leino atthe Institute for Heavy IonResearch at Darmstadt, Ger., 1996 bombardment of lead with zinc ions20882Pb + 7030Zn ——> 277112 (0.3 ms) + 10n . . .See as table:
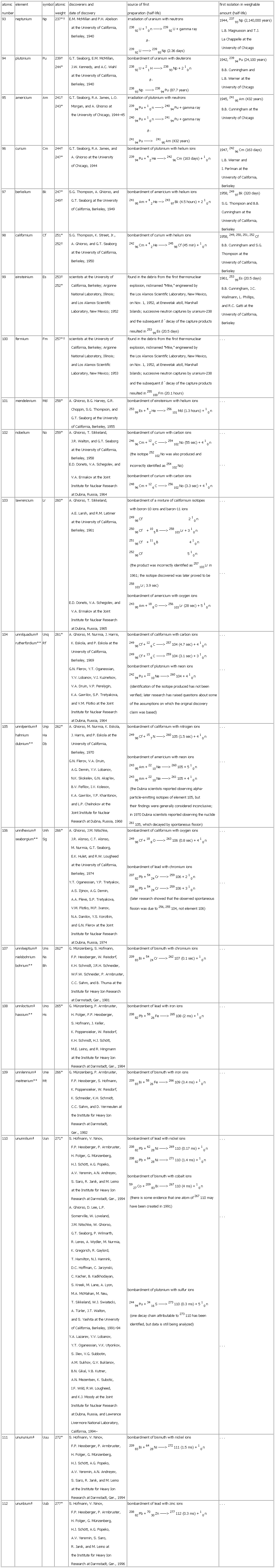 *Mass number of longest-lived isotope. †Mass number of more available isotope. ‡Temporary systematic name assigned by the International Union of Pure and AppliedChemistry (IUPAC) until a permanent name has been approved. Name recommended by the American Chemical Society Committee on Nomenclature in 1994.**Name recommended by the IUPAC in 1997.
*Mass number of longest-lived isotope. †Mass number of more available isotope. ‡Temporary systematic name assigned by the International Union of Pure and AppliedChemistry (IUPAC) until a permanent name has been approved. Name recommended by the American Chemical Society Committee on Nomenclature in 1994.**Name recommended by the IUPAC in 1997.* * *
Universalium. 2010.
